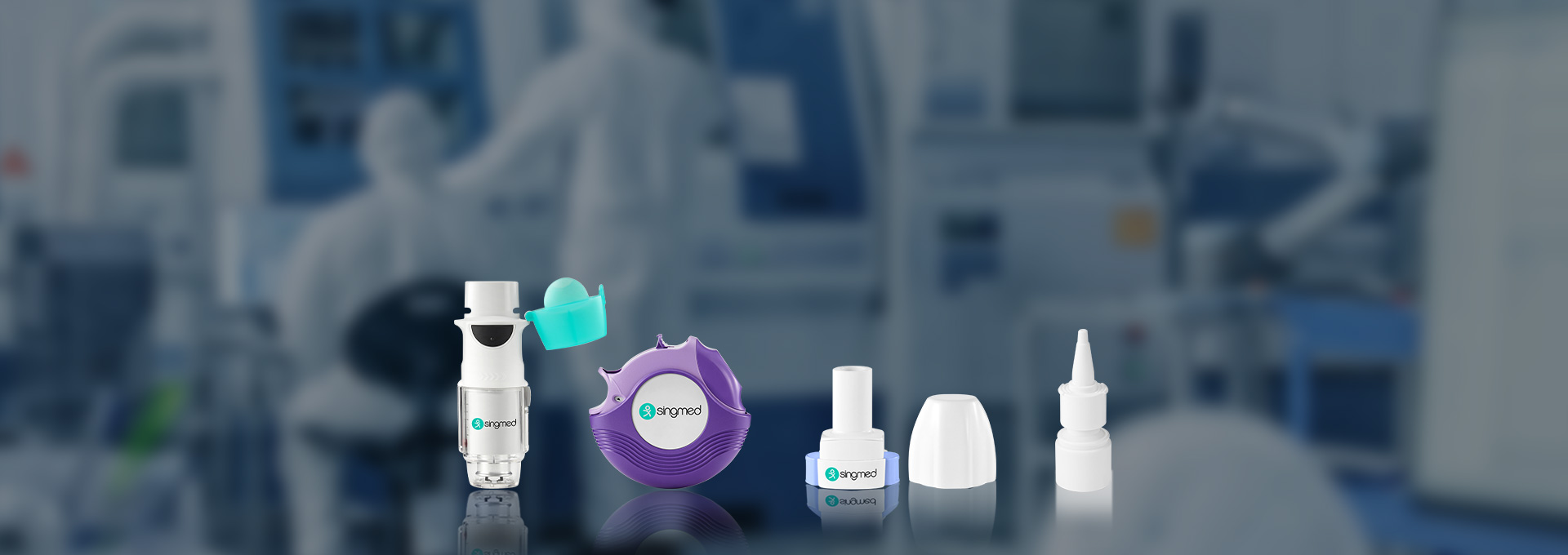
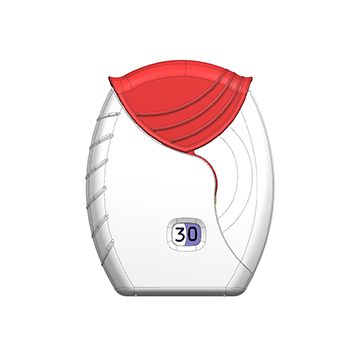
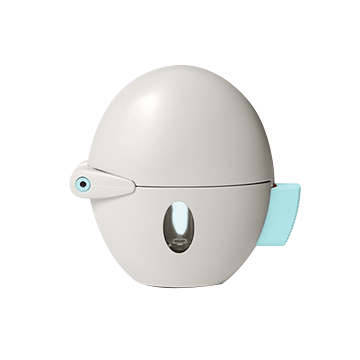
Dry Powder Inhaler is a widely appplied inhaled delivery dosage form which is used by a large number of patients for the delivery of drug to treat asthma and chronic obstructive pulmonary disease. The advantages of DPI include portability, ease of operation, quick administration, and minimal throat irritation.
The DPI offers excellent patient convenience, particularly for combination therapies, and also better compliance. The design and development of any powder drug-delivery system is a highly complex task.
The device addresses the several limitations of existing inhalers through its innovative design and process, facilitating effortless inhalation, efficient lung deposition, as well as convenient handling and portability. This will provide a more effective, secure, and convenient treatment option for the majority of COPD patients. Following Singmed's exclusive upgrade, our self-developed SMI device is equipped with micro-channel silicon-based chips which enable full atomization of the active pharmaceutical ingredients. In comparison to other inhalation devices, this device's internal design allows for spray generation solely through mechanical means without any propellant. Consequently, patients have an extended period to inhale the drug and ensure it reaches their lungs effectively. This enables true substitution to the novel device.
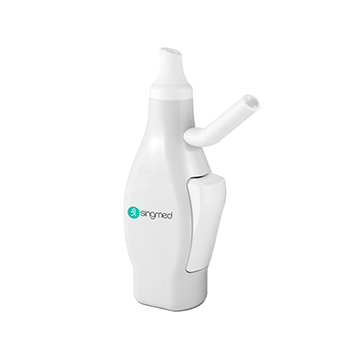
Singmed's nasal spray product matrix is centered on "Interface Standardization + Functional Modularity + Scenario Customization," covering the full lifecycle needs from generic drug to new drug development. With over 10 mature models and flexible R&D capabilities, we assist clients in accelerating product launch. We have built four core product series to achieve full-scene coverage from basic drug delivery to intelligent precision therapy.
· Nasal spray products rely on a silicon-based microfluidic atomization chip (patent pending), are compatible with mainstream filling machines such as Bosch and IMA, and have an annual production capacity of 50 million sets.
· The main body uses medical-grade polyolefin, complying with NMPA (China), FDA (USA), and CE-MDR (EU) requirements, providing global clients with full-cycle solutions from technical adaptation to mass production.
· The customized service process starts with demand analysis where clients provide vial interface drawings, drug formulations, and compliance checklists. Interface testing is completed within 72 hours with free samples, and engineering samples with atomization data are delivered within 5 working days.