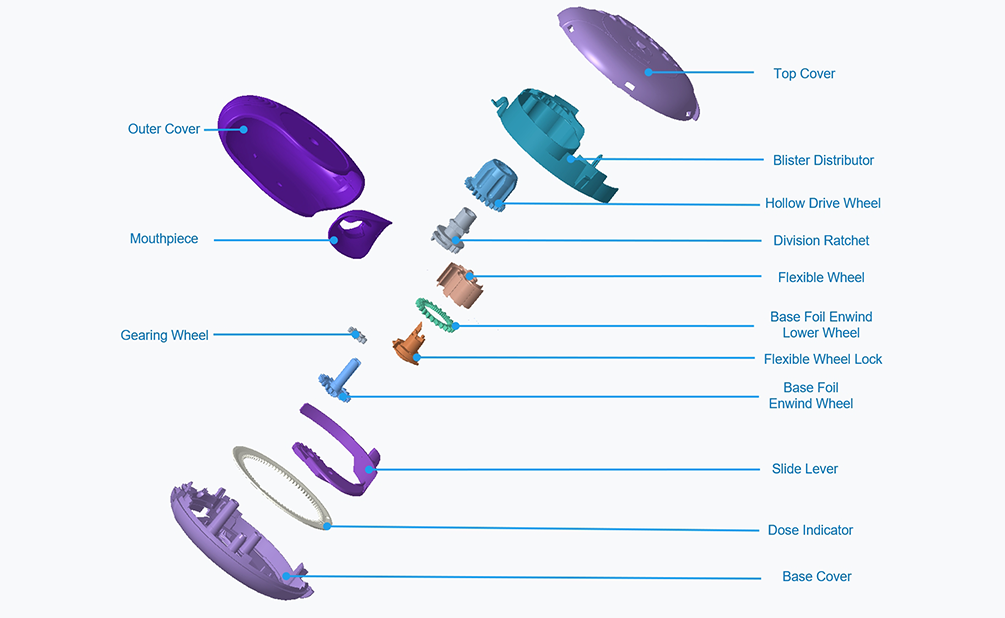
-
· Singmed self-developed product
· Multi-dose Blister DPI
· Singmed’s self-developed Diskus® devices (loaded with Umeclidinium Bromide and Vilanterol Trifenatate produced by GSK) have passed Bioequivalence Evaluation in 2022.
· Singmed’s self-developed Diskus® (loaded with Salmeterol Xinafoate and Fluticasone Propionate Powder for Inhalation produced by GSK) has been conductingBioequivalence researching with a number of Chinese leading listed pharmaceutical companies.
-
· With a distinctive product appearance, the atomization performance and the aerodynamic basis of our device are proved to be equivalent to the novel product, allowing for replacement of the patented device (Seretide® Diskus® produced by GSK). We are capable of providing customers with integrated customized services encompassing research and development, design, and production.
· Counting window for accurate indication of dosage
· Easy/Effortless compliance management. Suitable/ Appropriate for the elderly, Childrens and individuals with limited respiratory capacity.
· Inspiratory resistance range 0.03 to 0.06(kPa·s/L)
PRODUCTS
Drug Delivery Devices